Abstract
Introduction
Belantamab mafodotin is an antibody drug conjugate targeting B-cell maturation antigen (BCMA) on plasma cells and was the first BCMA-targeted drug approved by the FDA. Single agent effect was around 31-34% in a recent phase II study (Lonial et al, Lancet Oncology 2020). Side effects, including keratopathy and reduced visual acuity, necessitate dose reduction and dose delays that may limit efficacy.
Patients enrolled on clinical trials, however, often do not reflect a real-world population due to eligibility criteria that limit comorbidities and include wash-out periods not typical of clinical practice. The aim of this study was to assess response rates, dose modifications, and frequency of ocular adverse events in patients treated with belantamab mafodotin in a real-world setting.
Methods
All patients treated with commercial drug belantamab mafodotin at Memorial Sloan Kettering Cancer Center since October 2020 were included in the study. Descriptive statistics were used to assess patient characteristics, response rates, and rate of adverse events.
Results
Forty-two relapsed/refractory multiple myeloma patients were treated with belantamab mafodotin between October 2020 and the data cut off July 2021, including 55% (N=23) women; median age was 67 years. Twelve patients had been included in the Expanded Access Program with belantamab mafodotin prior to transitioning to commercial drug. Thirty patients (71%) had high risk cytogenetics, including gain 1q, t(4;14), t(14;16), t(14;20), complex karyotype, and MYC-translocations.
Patients had been treated with a median of 7 (range 4-14) prior lines of therapy. All patients had received an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody. Eleven patients had received a BCMA-targeted agent in clinical trials, including bi-specific antibodies, chimeric antigen receptor (CAR) T cells, and one patient had received prior trial therapy with belantamab mafodotin.
Patients received a median of 3 cycles (range 1-17) of belantamab mafodotin. The majority, 95% (N=40) as single agent, while two patients were treated with belantamab mafodotin in combination with other standard myeloma treatments.
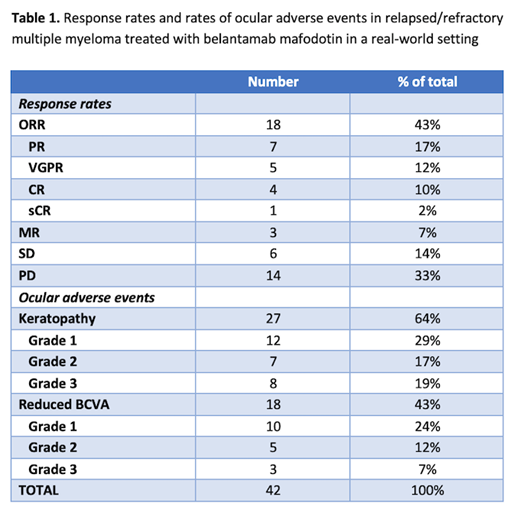
The overall response rate (ORR) in all patients was 43%; 18/42 patients. Of these, 7 achieved a partial response (PR); 5 patients achieved a very good partial response (VGPR), 4 patients achieved a complete response (CR) and 1 patient achieved a stringent CR (Table 1). Median duration of response for patients in the ORR cohort was 11 months (95% confidence interval: 9.1-not reached). Three patients achieved a minimal response (MR), 6 patients had stable disease (SD), and 1 patient was not evaluable for response assessment. After a median follow up of 7.5 months, 14 patients had progressive disease and 10 patients had died. Sixteen patients continued on belantamab mafodotin therapy at the time of data cutoff, July 31 st 2021.
In a separate analysis of patients not included in the Expanded Access Program, the ORR was 33%; 5 achieved a PR, 4 patients VGPR, 1 patient CR. Two patients achieved a MR, 3 patients had SD, and 14 patients had progressive disease.
Twenty-seven patients (64%) had any grade of ocular toxicity on ophthalmology exam. Using the Keratopathy and Visual Acuity (KVA) scale, 12 patients had grade 1 keratopathy, 7 had grade 2, and 8 had grade 3 keratopathy. Fourteen patients had presence of corneal microcysts. Eighteen patients experienced a decline in best corrected visual acuity (BCVA); 10 patients had grade 1, 5 had grade 2, and 3 patients had grade 3 decline in BCVA.
The dose of belantamab mafodotin was reduced from the starting dose of 2.5 mg/kg to 1.92 mg/kg in 17 patients: 16 patients due to ocular toxicity and 1 patient due to baseline cytopenia. Additionally, one or more doses were delayed in 9 patients due to keratopathy; the majority of patients (N=8) were able to continue therapy with maintained response on a lower dose after the delay.
Conclusion
In this heavily pre-treated multiple myeloma population, the ORR was 33-43% which is similar or better than reported in a recent phase II study. The rate of ocular toxicity (64% keratopathy) was also comparable to previous reports. These data demonstrate encouraging results for belantamab mafodotin treatment in the real-world setting with responses and toxicities comparable to clinical trial subjects. Additional patients and updated follow up will be reported at the meeting.
Hultcrantz: Intellisphere LLC: Consultancy; Curio Science LLC: Consultancy; Daiichi Sankyo: Research Funding; Amgen: Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Research Funding. Peterson: GlaxoSmithKline: Consultancy. Hassoun: Celgene, Takeda, Janssen: Research Funding. Korde: Medimmune: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding. Mailankody: Bristol Myers Squibb/Juno: Research Funding; Jansen Oncology: Research Funding; Physician Education Resource: Honoraria; Plexus Communications: Honoraria; Fate Therapeutics: Research Funding; Takeda Oncology: Research Funding; Allogene Therapeutics: Research Funding; Evicore: Consultancy; Legend Biotech: Consultancy. Landau: Takeda, Janssen, Caelum Biosciences, Celgene, Pfizer, Genzyme: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Genzyme: Honoraria. Shah: Amgen: Research Funding; Janssen Pharmaceutica: Research Funding. Lahoud: MorphoSys: Membership on an entity's Board of Directors or advisory committees. Scordo: McKinsey & Company: Consultancy; Angiocrine Bioscience: Consultancy, Research Funding; Omeros Corporation: Consultancy; Kite - A Gilead Company: Membership on an entity's Board of Directors or advisory committees; i3 Health: Other: Speaker. Landgren: Celgene: Research Funding; Janssen: Other: IDMC; Janssen: Research Funding; Janssen: Honoraria; Amgen: Honoraria; Amgen: Research Funding; Takeda: Other: IDMC; GSK: Honoraria. Giralt: CELGENE: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; AMGEN: Membership on an entity's Board of Directors or advisory committees; SANOFI: Membership on an entity's Board of Directors or advisory committees; Actinnum: Membership on an entity's Board of Directors or advisory committees; PFIZER: Membership on an entity's Board of Directors or advisory committees; JANSENN: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; JAZZ: Membership on an entity's Board of Directors or advisory committees. Lesokhin: Trillium Therapeutics: Consultancy; Behringer Ingelheim: Honoraria; bristol myers squibb: Research Funding; Genetech: Research Funding; Iteos: Consultancy; Janssen: Honoraria, Research Funding; pfizer: Consultancy, Research Funding; Serametrix, Inc: Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal